For the first time, the U.S. Food and Drug Administration on Thursday approved a treatment that can delay the onset of Type 1 diabetes.
Teplizumab, a monoclonal antibody that will be marketed under the brand name Tzield from pharmaceutical companies ProventionBio and Sanofi, is administered through intravenous infusion. The injection was shown in clinical trials to delay onset of insulin-dependent Type 1 diabetes for patients with autoantibody markers of early risk by over two years, with hopes for some that it can delay onset even longer.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said Dr. John Sharretts, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
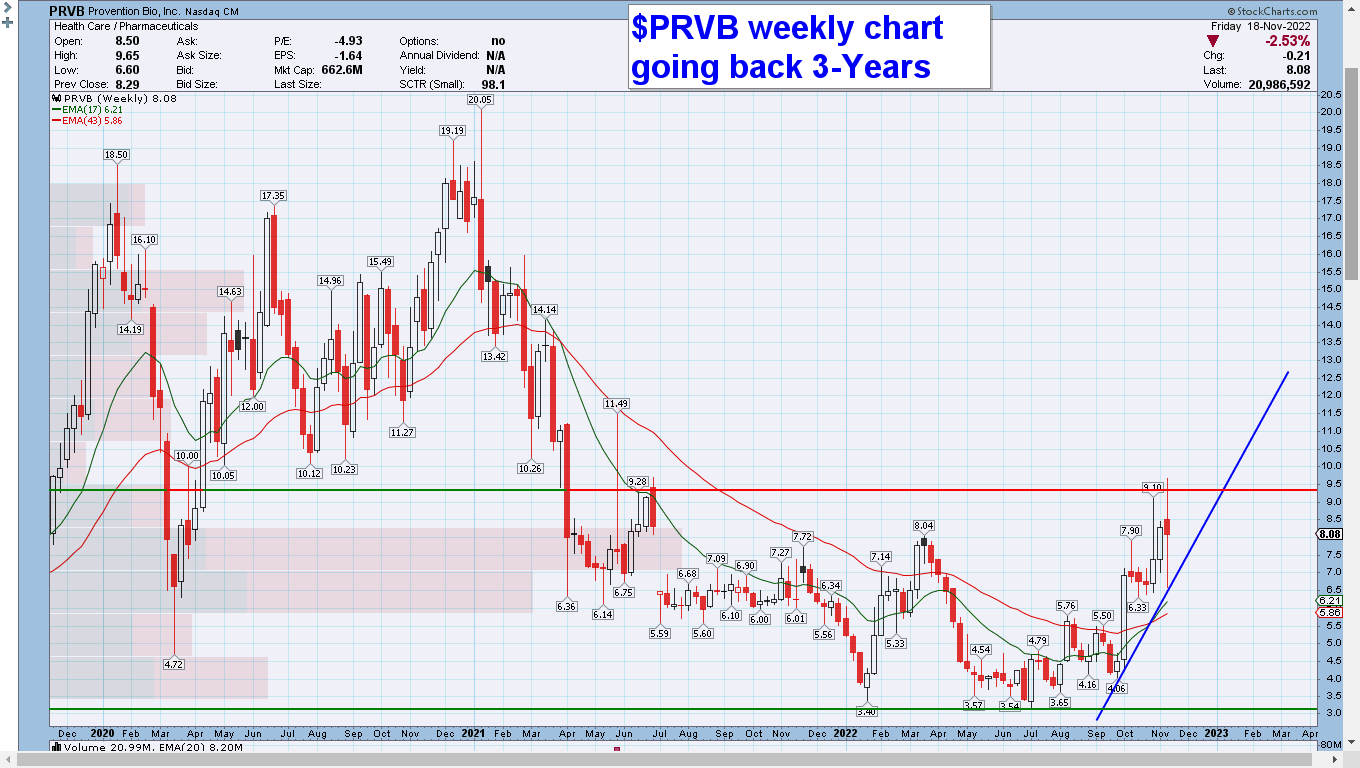
$PRVB daily, weekly, and monthly charts
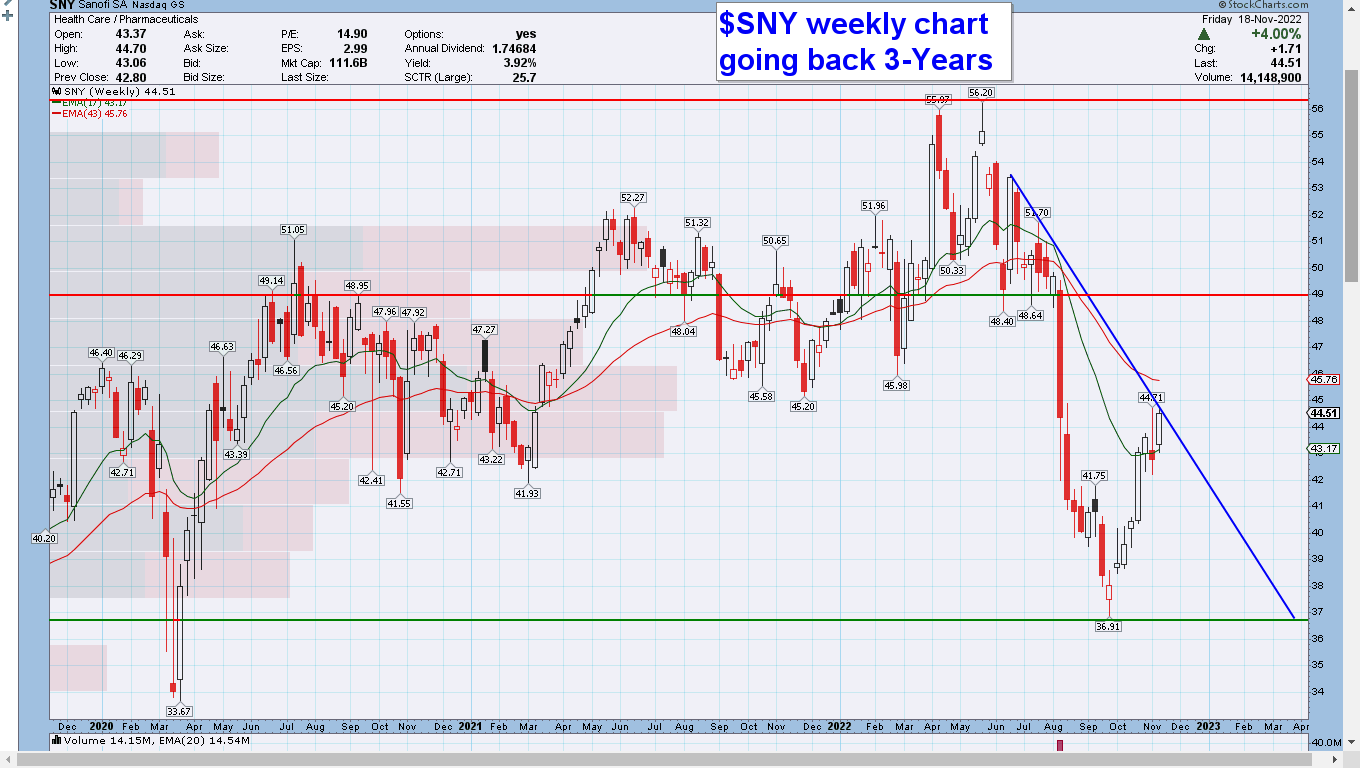
$SNY daily, weekly, and monthly charts
This headline answers the question: “How much is a vial of this Type 1 Diabetes Prevention drug?”