The Earth’s atmosphere is 78% nitrogen (N2, also known as dinitrogen) so we breathe nitrogen all day, every day.
Nitrogen is an extremely stable molecule because it has a triple bond between the two nitrogen atoms. The triple bond is extremely difficult to break. If it is broken by a chemical process the energy it releases when it is reformed is very high.
HMX, currently the most powerful chemical explosive known, releases four N2 atoms and four CO2 atoms when it explodes.
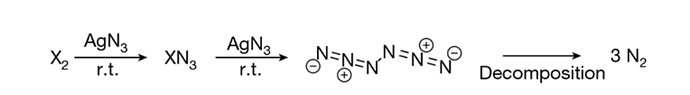
Today’s news describes the synthesis of N6 for the first time. This linear molecule releases three nitrogen N2 molecules when it explodes.
Most energetic molecule ever made is stable – in liquid nitrogen
By Tim Wogan, Chemistry World, 13 June 2025
The first neutral nitrogen molecule – other than dinitrogen – has been isolated and characterised by researchers in Germany. The molecule, the most energetic ever synthesised, is effectively stable at liquid nitrogen temperatures, which could make it attractive as an energy storage material.…
The weakest point of the structure – the ‘Achilles heel’ – is the bond between the two azide units. ‘It’s more like two times N3 than three times N2,’ explains Mardyukov. This increases the molecule’s lifetime at room temperature to around 36 milliseconds – long enough for it to be trapped and cooled to liquid nitrogen temperatures, where the researchers calculate its half life to be over 100 years.…
In the case of the neutral molecular nitrogen allotrope, N6, the activation barriers are relatively small (26 and 15 kcal/mol) and the energy difference, relative to N2, is huge (185 kcal/mol)…
When hexanitrogen does break down, it releases double the energy per unit mass of HMX – currently the most powerful chemical explosive known. Unlike some explosives that can leave residual pollutants such as nitrates, the only product is dinitrogen. …
[end quote]
This is extremely exciting for chemists because it’s a whole new type of molecule. But it may actually be of Macroeconomic significance because it’s stable at liquid nitrogen temperature which makes it extremely energy-dense. (Very dangerous if it warms up, of course.) The product of decomposition is stable, non-corrosive N2 and nothing else.
WOW!
Wendy